By Andrew Plummer
Nanotechnology is has an amazing way of influencing many different areas of science and technology. Particularly, reducing particles to nanoscale dimensions enables formation of energetic materials (explosives) from non-traditional ingredients normally considered unsuitable for these applications. This allows for the replacement of traditional ingredients, which are often highly toxic.
Take for example the use of calcium iodate as the oxidising agent in thermite mixtures with aluminium and boron [1]. On its own calcium iodate is a rather poor oxidiser. However, by ball milling it in mixtures of aluminium or boron powders to form agglomerates of ≈800 nanometres in diameter, the composite can now react so effectively to be able to explode with a shower of sparks and hot gasses (Figure 1). Importantly, Wang et al. [1] found that the boron and calcium iodate composite ignited at ≈325 °C, far below the melting point of either of the two ingredients.
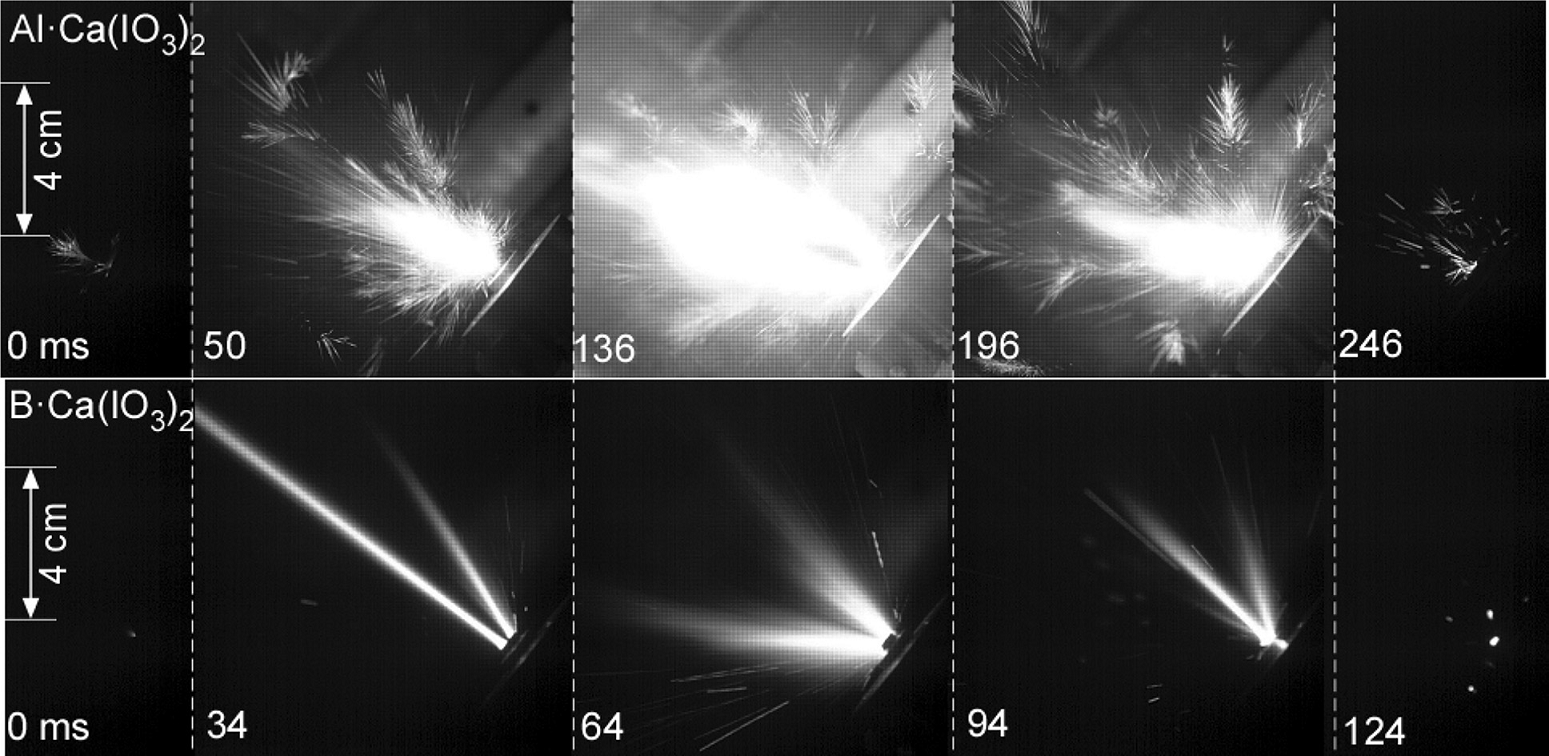
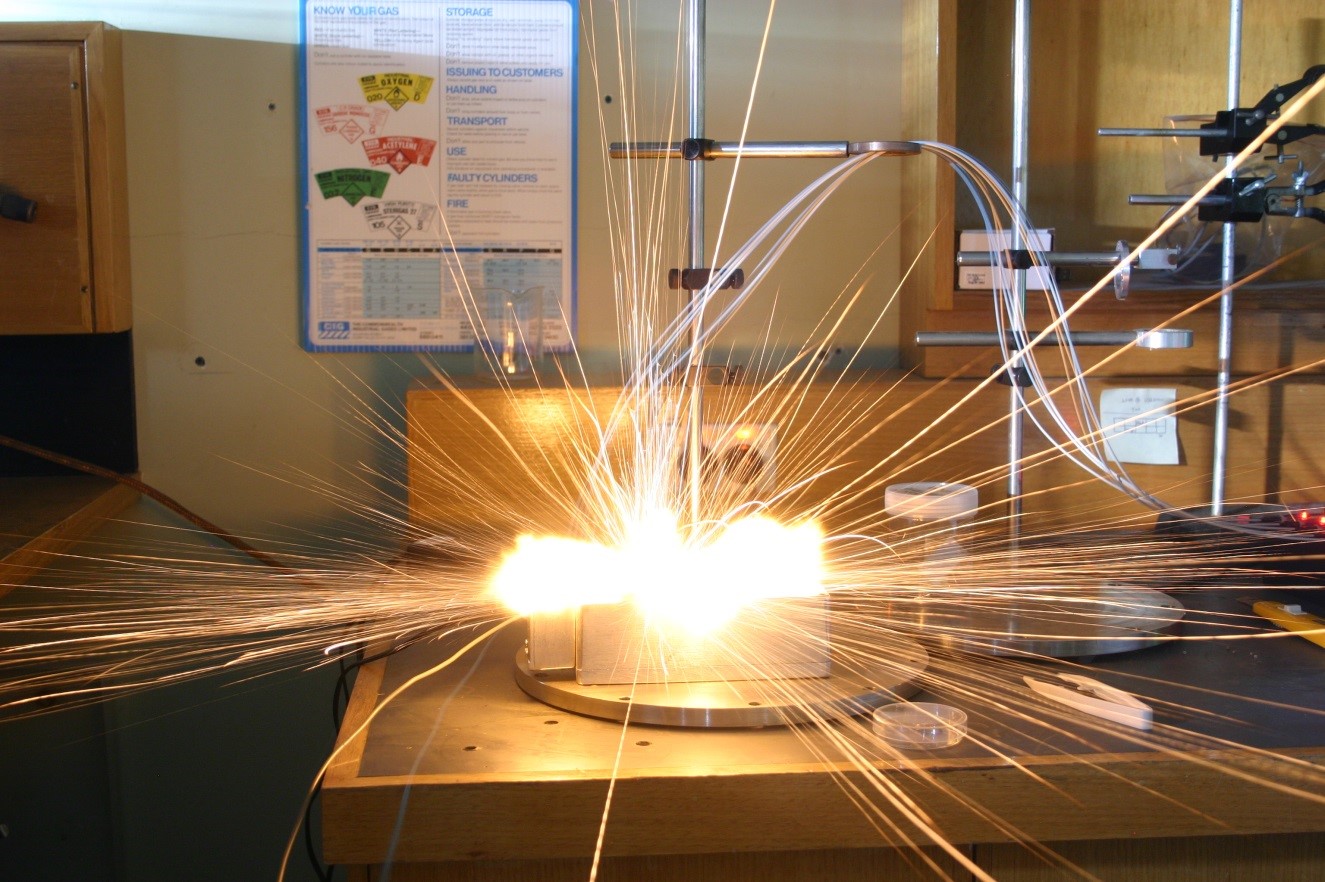
Such behaviour is only possible when the ingredients are mixed at the nanoscale. Solid phase diffusion reactions between the reactants is the first step to enable these reactions to occur below the melting or decomposition temperatures. Larger scale particles limit contact between the reactants, permitting any generated heat to conduct away quickly and preventing an energetic reaction. With particles sizes at the nanoscale however, small local reactions are able to cascade up to rapidly produce impressive results. By employing nanotechnology techniques to manufacture materials with controlled morphologies, researchers are able to use a far wider range of ingredients, leading to safer explosives for a range of applications.
References:
[1] Wang, S., Liu, X., Schoenitz, M., and Dreizin, E.L. (2017), “Nanocomposite thermites with calcium iodate oxidizer,” Propellants, Explosives, Pyrotechnics, Vol 42, pp 284-292.
[2] Plummer, A., Kuznetsov, V., Gascooke, J., Shapter, J., and Vowlcker, N. (2017) “Combined thermal and FTIR analysis of porous silicon based nano-energetic films,” RSC Advances, Vol 7, pp 7338 – 7345.

