Flinders University researchers have played a key role in a discovery that paves the way for broad use of a highly effective but expensive prostate cancer drug, potentially leading to cheaper treatment that requires lower dosage yet delivers the same benefits with less side effects.
Abiraterone works by blocking the production of testosterone, which prostate cancer cells need to thrive. It is proven to be effective at keeping prostate cancer under control but is usually prescribed only when other therapies have stopped working or for patients with advanced cancer, due to its high dosage requirements and corresponding costs and side effects.
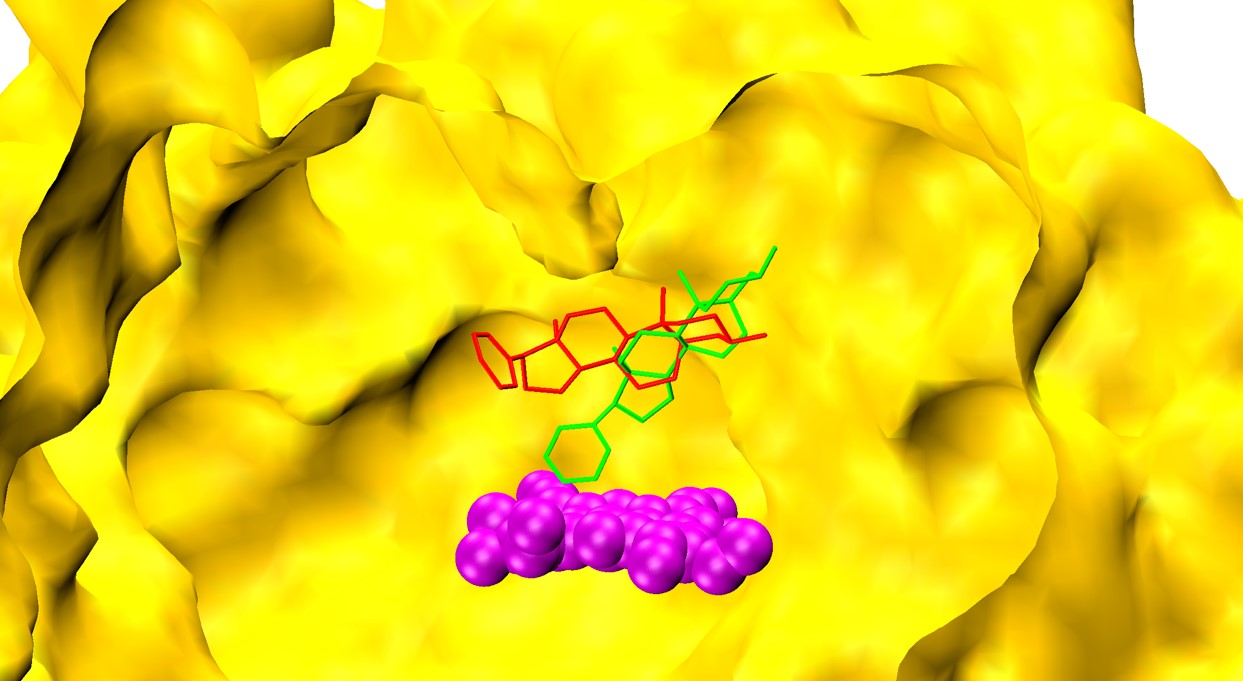
An international collaborative study has identified the novel binding mechanism of the cancer drug, whereby initial connection to its target within the cell is weak but strengthens over time to form a tight bind through a ‘two-step’ process.
Dr Pramod Nair and Professor John Miners of Flinders University worked with researchers at the National University of Singapore, the University of Chicago and the National Cancer Institute in the United States to uncover the process, following earlier analysis that revealed inconsistencies as to the nature and potency of the drug’s effect on its target cytochrome (P450 17A1 (CYP17A1).
The Flinders University researchers employed the high-performance computing and data services facility at The National Computational Infrastructure (NCI) in Canberra to model the enzyme CYP17A1 in the presence of abiraterone.
“A state-of-the-art molecular dynamics simulations technique was performed to gain atomic insights into the binding mechanism of abiraterone that were consistent with in vitro findings,” says Dr Pramod Nair, a Research Fellow in Computational and Molecular Pharmacology and partner on the study.
“These simulations allow conformational changes in proteins to be mapped, to gain insights into the dynamic behaviour of biological systems.”
The study describes the mechanism by which abiraterone binds to its target enzyme and induces a change in the protein, which further assists in tighter binding.
“Understanding how drugs interact with targets at an atomic level allows us to predict their binding mechanisms and provides a guide to the rational design of new therapeutic agents.” Dr Nair says.
“Using supercomputing platforms allows us to rapidly predict such mechanisms, reducing the five to eight years it would take on a regular computer to just three to four months.
“Understanding these processes in a short timeframe is valuable for dose optimisation, reducing adverse reactions, cost and improving patient care.”
The research paper, ‘Slow tight binding inhibition of CYP17A1 by abiraterone redefines its kinetic selectivity and dosing regimen’ (2020) by Cheong EJY, Nair PC, Neo RWY, Tu HT, Lin F, Chiong E, Esuvaranathan K, Fan H, Szmulewitz R, Peer CJ, Figg WD, Chai CLL, Miners JO, and Chan ECY was published in the Journal of Pharmacology and Experimental Therapeutics. DOI: https://doi.org/10.1124/jpet.120.265868

