Flinders University researchers want to expand their knowledge of massage pad use on legs – and you can be a helpful participant in the research if you are aged 50-75, healthy, and have a Body Mass Index between 25 and 30.
The Lymphoedema Clinical Research Unit at Flinders has received funding to gain a better understanding of the effects of vibrational massage on blood flow in the legs of healthy participants during and after treatment.
Each appointment is approximately 2 hours and is located at the Flinders Centre for Innovation in Cancer. The researchers will take a range of measurements including blood flow (sonography), leg volume and bio-impedance before and after treatment.
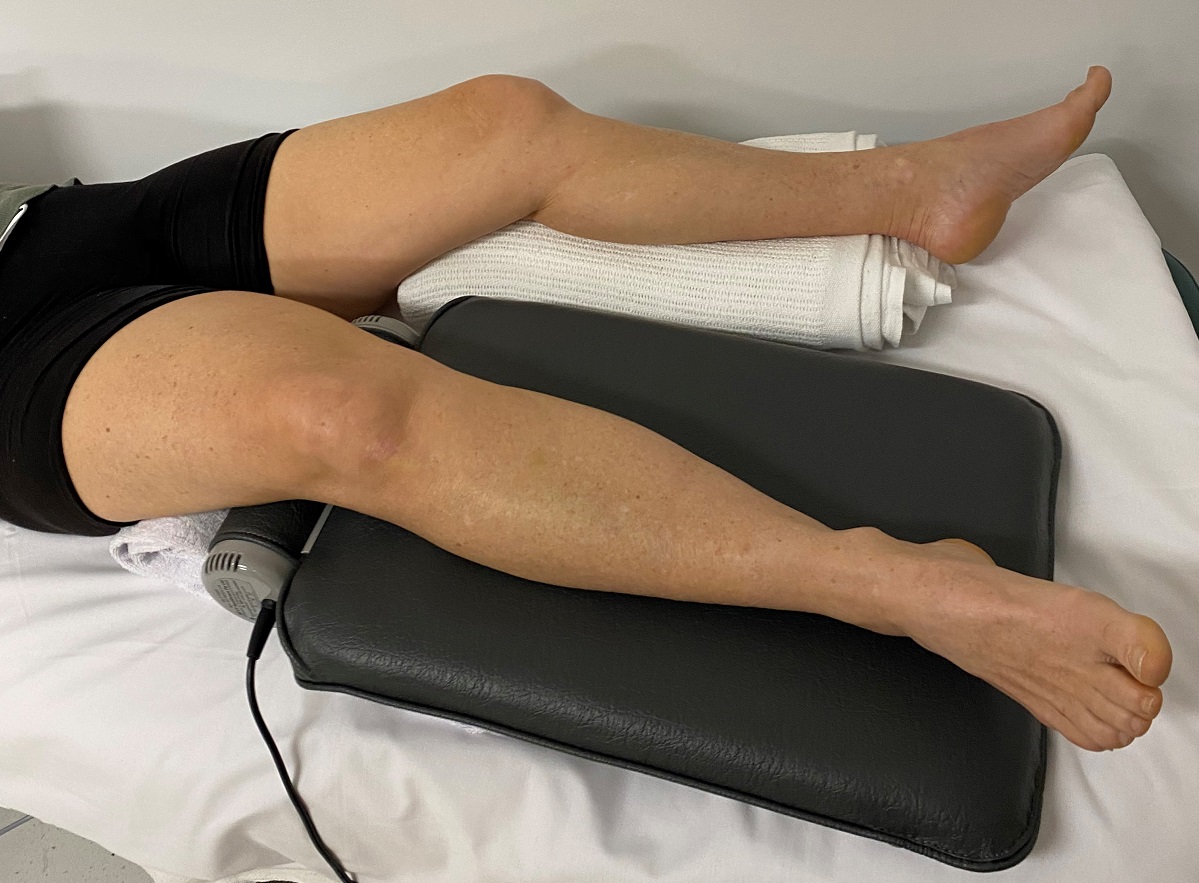
Treatment involves 3 x 10 minute applications of the Niagara massage pad to the lower back, the upper part and the lower part of your dominant leg, respectively. The placebo group will lie down for a similar time without treatment. Participants will have the opportunity to participate in both groups. Some clothing may be adjusted or removed to allow access to the whole leg. At all times you will be covered by a sheet or towel to maintain modesty.
Following your measurements, you will be given a comprehensive readout and explanation of your BMI, fluids, muscle and fat distribution like seen in body composition analysers at a gym.
As a gesture of thanks for your for your appointment, participants will be given a $15 voucher, redeemable at the FCIC café.
If you do not have an annual parking permit, you will be reimbursed for your parking costs.
If you’d like to be involved in this research, please email neil.piller@flinders.edu.au or LCRU@flinders.edu.au for a Participant Information Sheet, or leave your contact details on the LCRU message bank on 82044903 and a staff member will contact you.
Please note that exclusion criteria for the tests are: No prior surgery or radiotherapy associated with cancer or other treatment in the abdominal, groin or leg areas; oedema or lymphoedema in legs; no history of peripheral vascular disease; no Type 1 or 2 diabetes or no admission if you have been a smoker in the past 5 years.
This study has been reviewed and approved by the Southern Adelaide Clinical Human Research Ethics Committee # 2021/HRE00109. For further information regarding the ethical approval of the project the Executive Officer of the Committee can be contacted by telephone on 82046453 or by email health.SALHNOfficeforResearch@sa.gov.au

